What Is the Chemical Formula for Cotton
Cotton for Nonwovens Technical Guide
Cotton Morphology and Chemistry
Cellulose Chemistry
After scouring and bleaching, cotton is 99% cellulose. Cellulose is a macromolecule –– a polymer made up of a long chain of glucose molecules linked by C-1 to C-4 oxygen bridges with elimination of water (glycoside bonds). The anhydroglucose units are linked together as beta-cellobiose; therefore, anhydro-beta-cellobiose is the repeating unit of the polymer chain (see Figure 5 ). The number of repeat units linked together to form the cellulose polymer is referred to as the "degree of polymerization."
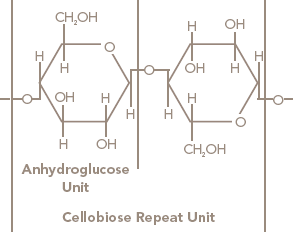
Figure 6 Chemical Structure of Cellulose
Wood pulp, rayon and cellophane (all three derived from wood cellulose) are also constructed of cellulose polymers. Cotton cellulose differs from wood cellulose primarily by having a higher degree of polymerization and crystallinity. Crystallinity indicates that the fiber molecules are closely packed and parallel to one another (as illustrated in Figure 6 ). Table 5 (see page 24) shows the average degree of polymerization and the average crystallinity of the cellulose fibers cotton, viscose rayon and wood pulp. Higher degree of polymerization and crystallinity are associated with higher fiber strengths.
The cellulose chains within cotton fibers tend to be held in place by hydrogen bonding. These hydrogen bonds occur between the hydroxyl groups of adjacent molecules and are most prevalent between the parallel, closely packed molecules in the crystalline areas of the fiber.
The three hydroxyl groups, one primary and two secondary, in each repeating cellobiose unit of cellulose are chemically reactive. These groups can undergo substitution reactions in procedures designed to modify the cellulose fibers or in the application of dyes and finishes for crosslinking. The hydroxyl groups also serve as principal sorption sites for water molecules. Directly sorbed water is firmly chemisorbed on the cellulosic hydroxyl groups by hydrogen bonding.
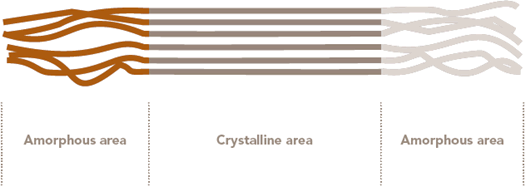
Figure 7 Amorphous and crystalline Areas of Polymers
Of particular interest in the case of cellulose fibers is the response of their strength to variations in moisture content. In the case of regenerated and derivative cellulose fibers, strength generally decreases with increasing moisture content. In contrast, the strength of cotton generally increases with increased moisture. This difference among fibers in their response to moisture is explained in terms of intermolecular hydrogen bonding between cellulose chains and their degree of crystallinity (see Tables 5 and 6 ).
| Table 5 Degree of polymerization and crystallinity of cellulose fibers | ||
| Fiber | Average Degree of Polymerization* | Average Crystallinity (%)** |
| Cotton | 9,000–15,000 | 73 |
| Viscose rayon Regular | 250–450 | 60 |
| High tenacity | 500–650 | |
| High wet modulus | 400–550 | |
| Wood pulp | 600–1,500 | 35 |
* Joseph, M., Introduction to Textile Science, 5th Edition, 1986.
** Shirley Institute; measured by X-ray diffraction.
Thermoplastic fibers melt at elevated temperatures and have a glass transition temperature at some point below the polymer's melting point. At the glass transition temperature, a thermoplastic fiber becomes brittle and loses its elasticity. Cotton is not a thermoplastic fiber; therefore, it has no glass transition temperature and remains flexible even at very low temperatures. At elevated temperatures, cotton decomposes instead of melting. Long exposure to dry heat above 300°F (149°C) causes cotton fibers to decompose gradually, and temperatures above 475°F (246°C) cause rapid deterioration.
| Table 6 Dry and wet strengths of fibers (g/tex) | ||
| Fiber | Dry | Wet |
| Cotton | 27–45 | 30–54 |
| Rayon (regular) | 22–27 | 10–14 |
| Wood pulp | 27–54 | 27–54 |
Cotton's Unique Fiber Morphology
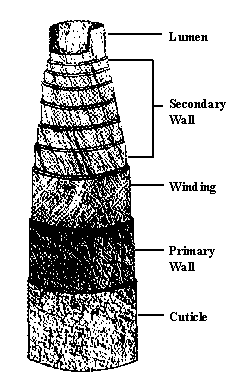
Figure 8 Structure of a cotton fiber
A mature cotton fiber has the following six parts.
The "cuticle" is the outer waxy layer, which contains pectins and proteinaceous materials. It serves as a smooth, water-resistant coating, which protects the fiber. This layer is removed from the fiber by scouring.
The "primary wall" is the original thin cell wall. Mainly cellulose, it is made up of a network of fine fibrils (small strands of cellulose). This makes for a well-organized system of continuous, very fine capillaries. It is well known that fine capillaries rob liquids from coarse capillaries. The fine surface capillaries of each cotton fiber contribute greatly to cotton's wipe-dry performance.
The "winding layer" (also called the S1 layer) is the first layer of secondary thickening. It differs in structure from both the primary wall and the remainder of the secondary wall. It consists of fibrils aligned at 40 to 70-degree angles to the fiber axis in an open netting type of pattern.
The "secondary wall" (also called the S2 layer) consists of concentric layers of cellulose, which constitute the main portion of the cotton fiber. After the fiber has attained its maximum diameter, new layers of cellulose are added to form the secondary wall. The fibrils are deposited at 70 to 80-degree angles to the fiber axis, reversing angle at points along the length of the fiber. The fibrils are packed close together, again, forming small capillaries.
The "lumen wall" (also called the S3 layer) separates the secondary wall from the lumen and appears to be more resistant to certain reagents than the secondary wall layers.
The "lumen" is the hollow canal that runs the length of the fiber. It is filled with living protoplast during the growth period. After the fiber matures and the boll opens, the protoplast dries up, and the lumen naturally collapses, leaving a central void, or pore space, in each fiber.
Figure 8 shows a schematic structure of a mature cotton fiber, identifying its six parts.
Throughout the fiber structure, there are variously sized pores or capillary spaces between the variously sized fibrils in each of the six fiber parts. Thus, the cotton fiber can be viewed as a microscopic physical sponge with a complex porous structure. This internal structure makes cotton fibers accessible to liquids and vapors. The capillary action of the fibrils pulls liquid in, where it is held in pores between the fibrils. This structure accounts for cotton's wickability and unique absorbing capacity.
The cotton fiber, when observed in its entirety, is a flat, twisted ribbon, with 50 to 100 convolutions per inch. The fiber is tapered on one end and fibrillated on the other, where it was joined to the cottonseed. This provides the fiber with a soft touch or feel, because it has no sharply cut ends, as do synthetic staple fibers.
Select the Right Fiber
Some absorbent nonwoven products, such as surgical sponges, sanitary napkins, tampons and cosmetic pads and puffs can be satisfactorily made from by-product cotton fiber (gin motes, comber noils and other mill waste). Most of these products evolve from a bleached cotton coil (an oversized sliver) that needs little integrity (fiber-to-fiber cohesion). However, roll goods from lightweight webs made by carding or air-forming require textile-grade fiber. Table 7 shows recommended fiber properties, testing methods and performance criteria for manufacturing nonwoven roll goods that can be used in cover stock for diapers and feminine pads or as fabrics for the protective clothing and home product areas.
| Table 7 Properties of bleached cotton for nonwoven roll goods | ||
| Property | Criterion | Test Method |
| Micronaire | ≥ 4.9 | HVI |
| Length | ≥ 0.95 in. | HVI |
| Length uniformity | ≥ 81.0% | HVI |
| Strength | ≥ 23.0 g/tex | HVI |
| Non-lint content | ≤ 0.8% | MDTA-3 |
| Fiber-to-fiber cohesion | ≤ 1,700 g | ICI fiber cohesion test |
| Fiber openness | ≥ 100 cc/g | ITT test method |
Fiber length and strength are important in the manufacture of lightweight roll goods, for obvious reasons. However, good fabric appearance is more important than fabric strength in certain nonwoven products, and fiber micronaire is a major consideration for these items. An undesirable characteristic for such items is high nep content. High-micronaire cotton tends to have lower nep content after ginning and is less prone to form additional neps in subsequent processing.
To study the influence of micronaire on nep formation, three bales of cotton of both high and low micronaire were selected, based on HVI data. Care was taken to keep other fiber variables constant to obtain a true measure of micronaire's influence on nep formation. The bales were bleached at a commercial operation using normal production procedures, and the nep content at various stages of processing was determined with the Zellweger Uster AFIS nep tester.
Table 8 compares the properties and nep content of the two groups of cotton. The high-micronaire cotton showed some increase in nep content due to bleaching, most likely attributable to the fiber opening stages before and after drying. In contrast, the low-micronaire cotton showed substantial increases in nep content during bleaching and during nonwoven web formation. The benefits of using a higher micronaire cotton are dramatic.
| Table 8 Influence of micronaire on nep formation during processing | ||
| Property | High Micronaire | Low Micronaire |
| Micronaire | 4.5 | 3.0 |
| Length (inches) | 1.0 | 1.0 |
| Length uniformity (%) | 79.4 | 78.8 |
| Strength (g/tex) | 25.0 | 25.0 |
| Elongation (%) | 7.0 | 7.4 |
| Neps per gram: | ||
| Original bale | 375 | 574 |
| Mechanically cleaned | 354 | 860 |
| Bleached | 520 | 1140 |
| Card web | 540 | 2406 |
What Is the Chemical Formula for Cotton
Source: https://www.cottoninc.com/quality-products/nonwovens/cotton-fiber-tech-guide/cotton-morphology-and-chemistry/
0 Response to "What Is the Chemical Formula for Cotton"
Post a Comment